
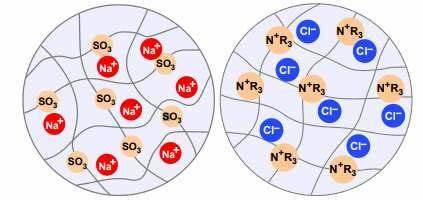
Ion exchange resin is a solid polymer compound with functional groups (composed of active ions and fixed ions), a network structure, and is insoluble in acids, alkalis, and organic solvents. It is usually a spherical particle. Its polymer active group is generally a polyacid or polybase. Therefore, from the electrochemical point of view, ion exchange resin is an insoluble multivalent dielectric. The unit structure of ion exchange resin consists of three parts: a cross-linked network skeleton with a three-dimensional spatial structure, a functional group connected to the skeleton (active group, such as -SO3-, -N(CH3)3-) and an active ion with an opposite charge to the active group (i.e., an exchangeable ion, such as H+, OH-). The inert and insoluble network skeleton and the active group are integrated and cannot move freely. The active ions can migrate freely between the network skeleton and the solution.
Ion exchange resin relies on the counterions dissociated from the functional groups to exchange with the ions in the solution. The driving force of the exchange process is the concentration difference between the two ions and the difference in the affinity of the functional groups to the free ions.
When the resin is in a solution, the active ions on it can exchange with the same ions in the solution according to the different chemical affinity with the functional groups of the resin. This exchange is carried out in equivalent amounts.
The polymer active group is the factor that determines the main performance of the ion exchange resin. If the active group releases positively charged active ions, this ion exchange resin can exchange with other cations in the solution and is called a cation exchange resin; if the released active ions are anions, this ion exchange resin can exchange anions in the solution and is called an anion exchange resin.
The ion exchange reaction is reversible, and the ion exchange resin can be reused repeatedly through regeneration. In addition to the exchange effect, ion exchange resins also have multiple functions such as selection, adsorption, catalysis and decolorization. At present, they have been widely used in water treatment, metal smelting, atomic energy science and technology, marine resource development, chemical production, sugar refining, food processing, medicine and health, analytical chemistry and environmental protection.
Ⅰ. Classification of ion exchange resins
1. Classification by the nature of the active group
According to the nature of the active group, ion exchange resins can be divided into cation exchange resins and anion exchange resins.
Among them, the exchange resin with acidic active groups that can exchange with cations in water is called cation exchange resin; the exchange resin with alkaline active groups that can exchange with anions in water is called anion exchange resin.
According to the strength of H+ or OH- ionization on the active group, it can be divided into strong acid cation exchange resin and strong basic anion exchange resin, weak acid cation exchange resin and weak basic anion exchange resin.
2. According to the classification of pore type,
It can be divided into gel resin, macroporous resin and uniform porous resin.
3. According to the type of matrix,
Ion exchange resin can also be divided into styrene series, acrylic series, phenolic series, epoxy series and vinylpyridine series according to the type of matrix.
Ⅱ. Basic types of ion exchange resins
1. Strong acid cation exchange resins
The active groups of this type of resin are strong acid groups [sulfonic acid groups (-SO 3 H) or methylene sulfonic acid groups (-CH 2 SO 3 H) ], which have a high degree of ionization and are not affected by changes in solution pH. They can undergo ion exchange reactions in the pH range of 1 to 14.
Strong acid resins have a weak binding force with H+, so it is difficult to regenerate the hydrogen form, so the acid consumption is relatively large.
2. Weakly acidic cation exchange resin
Including carboxyl -COOH, oxyacetic acid group -OCH 2 COOH , phenolic hydroxyl group C 6 H 5 OH - and β-diketone group -COCH 2 COCH 3. They are all weakly acidic groups, and their degree of ionization is greatly affected by changes in the pH of the solution. Almost no exchange reaction occurs in acidic solutions. Their exchange capacity decreases with the decrease in the pH of the solution and increases with the increase in the pH value.
Carboxylic acid cationic resins must work normally in a solution with a pH>7. For phenolic hydroxyl esters with weaker acidity, they should react in a solution with a pH>9.
Contrary to the exchange properties of strong acidic cations, the binding force between H+ and weakly acidic cation resins is very strong, so it is easy to regenerate into hydrogen form, and the acid consumption is also small.
3. Strongly basic anion exchange resins
The active group of this type of resin is a quaternary ammonium group, with a trimethylamine group RN + (CH 3 ) 3 OH - (Type I) and dimethyl-B-hydroxyethylamine group RN + (CH3) 2 (C 2 H 4 OH)OH - (Type II).
Similar to strong acid ion exchange, its active group has a strong degree of ionization and is not affected by changes in solution pH. It can be used in the pH range of 1~14. Its exchange reactions are:
This type of resin is more stable than the hydroxyl type when it is in chlorine form and has better heat resistance. Therefore, most of the products are sold in chlorine form.
The thermal stability, oxidation resistance, mechanical strength and service life of Type I resin are better than those of Type II resin, but it is more difficult to regenerate. Type II resin is better than Type I in terms of organic pollution resistance, and Type II resin is also weaker in alkalinity than Type I.
Due to the weak binding force between OH - and strong base exchange resin, the amount of regeneration agent NaOH is large. This type of resin is mainly used to prepare salt-free water (removing weak acid radicals such as SiO2- and CO32-.
4.Weakly basic anion exchange resin
The active groups of this type of resin include primary amine groups -NH2 , secondary amines -NHR and tertiary amines -N (R) 2 , as well as pyridine C6H5N and other groups.
The degree of ionization of the group is weak, and like weakly acidic cation resins, the exchange capacity is greatly affected by changes in solution pH. The lower the pH, the higher the exchange capacity, and vice versa. Therefore, it is used in solutions with pH <7. The exchange reactions are similar to weakly acidic cation resins, the salt RN + H3Cl- generated by weakly basic anion exchange resins is easily hydrolyzed into RN + H3OH- , which also shows that OH- has a strong binding force, so it is easier to regenerate the hydroxyl type with NaOH, and the alkali consumption is also small, and it can even be regenerated with Na2CO3 .
Ⅲ. Adsorption selection of ion exchange resins
Ion exchange resins have different affinities for different ions in solution and are selective in their adsorption. There is a general rule for the strength of the exchange adsorption of various ions by resins, but different resins may have slight differences.
Generally, resins with high crosslinking degree have stronger selectivity for ions, and resins with macroporous structure have lower selectivity than gel-type resins. This selectivity is greater in dilute solutions and smaller in concentrated solutions.
Ⅳ. The main functions of ion exchange resin
1. Ion exchange
The most basic function of ion exchange resin is ion exchange.
When the resin comes into contact with the electrolyte solution, the counter ions inside the resin particles dissociate and undergo an ion exchange reaction with the ions in the solution that have entered the resin.
Ion exchange reactions are usually reversible equilibrium, and the direction of the reaction is affected by factors such as the properties of the resin exchange groups, the properties and concentration of the ions in the solution, the pH value of the solution, and temperature.
By utilizing this reversible equilibrium property, ion exchange resins can be regenerated and reused repeatedly. However, for chelating resins and resins that have a high selectivity for certain ions, the exchange reaction is generally irreversible, and other methods must be used to desorb the exchanged ions.
2. Dehydration
The exchange groups in ion exchange resins are strongly polar and hydrophilic, so the dried resin has a strong water absorption effect. Dry strong acid cation exchange resins can be used for dehydration of various organic solvents.
3. Catalysis
Ion exchange resin is a high molecular weight acid or base, so it catalyzes some organic chemical reactions just like low molecular weight acids or bases. In particular, macroporous ion exchange resin has been widely used to catalyze esterification, alkylation, olefin hydration, acetalization, hydrolysis, dehydration (ring-opening) and comprehensive reactions.
The advantages of using ion exchange resin as a catalyst are that the reaction products and the catalyst are easy to separate, post-processing is simplified, and the resin is not corrosive to the equipment.
4. Decolorization
Pigments are mostly anionic or weakly polar and can be removed by ion exchange resins. In particular, macroporous resins have a strong decolorizing effect and can be used as excellent decolorizing agents. For example, ion exchange resins are very effective in decolorizing and refining glucose, sucrose, beet sugar, etc. Compared with activated carbon, it has the advantages of being reusable, long-term and easy to use.
5. Adsorption
Ion exchange resin has the function of adsorbing non-electrolyte substances from solution, which is similar to the adsorption behavior of non-ionic adsorbents. Its adsorption is reversible and can be desorbed by selecting appropriate solvents.
Macroporous ion exchange resins can not only adsorb weakly polar or non-polar substances from polar solvents, but also adsorb weakly polar substances from non-polar solvents, and can also be used as gas adsorbents.
Ⅴ. Application fields of ion exchange resins
1. Water treatment
The demand for ion exchange resins in the water treatment field is very large, accounting for about 90% of the output of ion exchange resins, and is used to remove various anions and cations in water.
At present, the largest consumption of ion exchange resins is used in pure water treatment in thermal power plants, followed by atomic energy, semiconductors, and electronics industries.
2. Food industry
Ion exchange resins can be used in industrial devices such as sugar production, monosodium glutamate, wine refining, and biological products.
For example: the manufacture of high fructose syrup is to extract starch from corn, then undergo hydrolysis reaction to produce glucose and fructose, and then undergo ion exchange treatment to produce high fructose syrup. The consumption of ion exchange resins in the food industry is second only to water treatment.
3. Pharmaceutical industry
Ion exchange resins in the pharmaceutical industry play an important role in the development of a new generation of antibiotics and the improvement of the quality of existing antibiotics. The successful development of streptomycin is a prominent example. In recent years, there have been studies on the extraction of traditional Chinese medicine.
4. Synthetic chemistry and petrochemical industries
Often use acids and bases as catalysts for esterification, hydrolysis, transesterification, hydration and other reactions in organic synthesis. Ion exchange resins can be used instead of inorganic acids and bases to carry out the above reactions, and they have more advantages. For example, the resins can be used repeatedly, the products are easy to separate, the reactors will not be corroded, the environment will not be polluted, and the reactions are easy to control.
5. Environmental protection
Ion exchange resins have been used in many environmental protection issues that are of great concern.
At present, many aqueous or non-aqueous solutions contain toxic ions or non-ionic substances, which can be recycled with resins. For example, metal ions are removed from electroplating waste liquid, and useful substances are recovered from film production waste liquid.
6. Hydrometallurgy and other
Ion exchange resins can separate, concentrate, purify uranium from depleted uranium mines, and extract rare earth elements and precious metals.
Extrepure Resin(Shanghai) CO.,LTD
